Mr. Miller
Thermodynamics
Latent Heat
As you add heat to water, the temperature rises. When the temperature reaches 100ºC, the water starts to boil, i.e. it starts to change from a liquid to a gas. If heat continues to be added, what happens to the water? Does its temperature continue to rise as the water boils away?
It turns out that the water temperature stays constant at the boiling point, even as heat is continually added to the water. This heat, which does not raise the temperature of the water any further does have an effect: it is the energy that causes the phase of the substance to change from liquid to gas, from water to steam.
In other words, some amount of energy is required for matter to change phase from liquid to gas. Each substance requires a very particular amount of energy, in the form of heat, to change 1 gram of the substance in liquid form into gaseous form. This amount is known as the latent heat of vaporization for that substance. Similarly, some heat is required to change 1 gram of the substance in solid form into liquid form. This amount is known as the latent heat of fusion for that substance.
Latent heat is latent because the heat added to the substance doesn’t raise its temperature. Rather, the energy added to the system only helps it to change its phase.
Water has the following values for latent heat:
- The latent heat of vaporization is 540 calories per gram. – changes 1g water at 100 ºC into 1g steam at 100 ºC
- The latent heat of fusion is 80 calories per gram. – changes 1g of ice at 0 ºC into 1g of water at 0 ºC
The process also works in reverse. Above, heat was added to the substance, changing its phase from solid to liquid or liquid to gas. What if we cool the substance instead?
In this case, heat is released as the substance changes phase from gas to liquid and liquid to solid. It makes sense that the amount of heat required to change a gram of substance from liquid to gas is the same amount that is released when the substance changes from a gas back into a liquid. Because the values are the same, and the only difference is the direction of the process, we only use a single term for each phase change:
Latent heat of vaporization: phase changes from liquid to gas OR gas to liquid
Latent heat of fusion: phase changes from solid to liquid OR liquid to solid
So we can define latent heat as the heat which flows to or from a material without a change to temperature. The heat will only change the structure or phase of the material. e.g. melting/freezing and boiling/condensing.
The following picture shows what happens to ONE gram (one ml) of water. The orange arrow on the top represents adding heat, from left to right, and the blue arrows represent releasing heat from right to left on the bottom.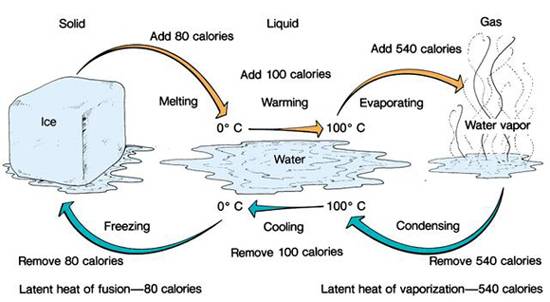
So, let’s look at the whole process together in the form of a graph. The graph describes how the temperature of 1 gram of water in its three forms (ice, water, steam) changes as heat is added or taken away.
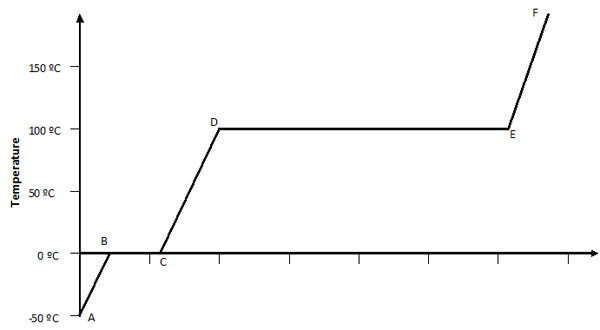
A to B: pure ice
B to C: a mixture of water and ice all at 0 ºC
C to D: pure water
D to E: a mixture of water and steam all at 100 ºC
E to F: pure steam