Sample Writeup for “Ice Melters” Demonstration:
Materials:
Two basically identical pieces of ice
A styrofoam block
A metal block
Procedure:
A piece of ice was placed on the styrofoam block and on the metal block at the same time. We watched to see which piece of ice would melt faster. The blocks had been in the same room together all night long.
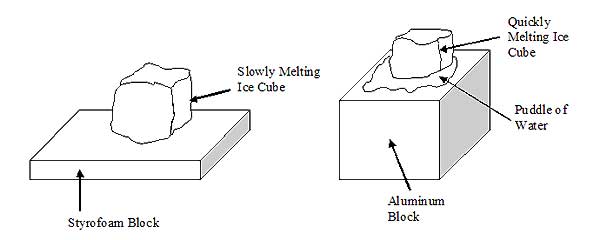
Observations and Data:
The ice that was placed on the styrofoam block took a very long time to melt – upwards of 15 minutes. It very slowly dripped into the styrofoam, which changed its color where the water was absorbed. The ice that was placed on the metal block started to melt immediately in a dramatic way as soon as it was in contact with the block. It melted entirely in only about 30 seconds to a minute. While it was melting it moved around on the top of the block and formed a large puddle of water.
After the ice had melted on the metal block, a student compared the temperature of the metal block with another, identical metal block that had also been sitting in the room all night but had not been in contact with the ice by touching them with his hands and comparing how hot or cold they felt. Lyle indicated that the block that had melted the ice was colder than the other block, but that both were much colder than the styrofoam block. Later we discovered that the temperature of the blocks was the same at the beginning: 68 degrees Fahrenheit. We knew this because we used temperature strips to check the temperature of each object.
Discussion and Analysis:
The metal block and styrofoam block are made of two different substances. Every substance gives and takes heat to or from surrounding objects with different speeds. A number is given to this property and it is called the “thermal conductivity” or “heat conductivity". Materials with low heat conductivity are considered “insulators”, meaning that they don’t easily give or take heat to or from surrounding objects. Materials with high heat conductivity are considered “conductors”, meaning that they easily give or take heat to or from surrounding objects.
Styrofoam has a very low heat conductivity, so it doesn’t do a good job of transmitting or conducting heat into the ice cube to melt it. Therefore it took a long time for heat to be transferred from the styrofoam block to the ice cube to melt it. The metal block has a very high heat conductivity, so it is easily able to give up its heat to the ice cube, which makes it melt faster. After the ice had melted on the metal block, it felt colder to the touch than the second metal block that hadn’t been in contact with the ice because it had given some of its own heat to the ice cube, so comparatively it was colder.
Conclusion:
Heat conductivity measures the rate at which heat can be transmitted through a substance. Therefore, the ice melted much faster on the metal block because of its high heat conductivity, while it melted slower on the styrofoam block because of its lower heat conductivity.